Bioreactor Reaction Monitoring Using QCL-Based Liquid Transmission Spectroscopy
Overview:
This application note demonstrates the effectiveness of Block Engineering's quantum cascade lasers (QCLs) in monitoring bioreactor chemistries using liquid transmission spectroscopy. Current non-destructive bioreactor monitoring methods, such as ATR-FTIR and Raman spectroscopy, are limited in their liquid penetration depths, and ATR-FTIR requires direct contact with the sample. Highly accurate methods, such as chromatography, mass spectrometry, and biosensors, are often destructive or require contact with the sample and cannot provide real-time measurements. QCLs offer a unique solution to bridge this gap with highly sensitive, in situ monitoring to accurately quantify chemical concentration levels.
 Block Engineering's QCLs are widely tunable mid- to long-wave infrared lasers. These compact lasers have diverse applications spanning personnel safety, academic research, manufacturing, and life sciences. QCLs are particularly effective in liquid transmission spectroscopy due to their high pulse powers and excellent signal-to-noise ratios. These qualities allow QCLs to measure chemical concentrations in water by observing absorption features with increased sensitivity and spectral resolution. Additionally, they operate in the infrared "fingerprint region" where many molecules exhibit unique absorption features, enabling identification of a wide range of chemicals. When paired with a Block Engineering MCT detector, QCLs offer a powerful solution for bioreactor monitoring, enabling highly sensitive, real-time, in situ analysis without contaminating or disturbing the bioreactor environment.
Block Engineering's QCLs are widely tunable mid- to long-wave infrared lasers. These compact lasers have diverse applications spanning personnel safety, academic research, manufacturing, and life sciences. QCLs are particularly effective in liquid transmission spectroscopy due to their high pulse powers and excellent signal-to-noise ratios. These qualities allow QCLs to measure chemical concentrations in water by observing absorption features with increased sensitivity and spectral resolution. Additionally, they operate in the infrared "fingerprint region" where many molecules exhibit unique absorption features, enabling identification of a wide range of chemicals. When paired with a Block Engineering MCT detector, QCLs offer a powerful solution for bioreactor monitoring, enabling highly sensitive, real-time, in situ analysis without contaminating or disturbing the bioreactor environment.
Procedure:
To provide proof of concept, QCL-based liquid transmission spectroscopy was used to correlate absorbance with chemical concentration levels of selected bioreactor-relevant chemicals. The target analytes — glucose, fructose, lactose, and glutamine — are commonly used in cell culturing and protein synthesis. Solutions of each chemical were prepared at concentrations ranging from 1mg/mL to 100 mg/mL in distilled water and injected into a Pike Technologies demountable liquid cell. This liquid cell consists of two 3 mm thick ZnSe windows separated by Teflon spacers with thicknesses ranging from 15 µm to 100 µm.
To perform measurements, a Block Engineering LaserTune™ benchtop QCL module was aligned to transmit an infrared beam through the liquid cell and into a Block MCT detector. The LaserTune used in this investigation features four QCLs that span the wavenumber range of 800 - 1800 cm-1. Absorbance spectra of the solutions were measured, and peak intensities were analyzed across concentration levels. According to the Beer-Lambert law, the relationship between absorbance and concentration is expected to be linear for a given path length.
Results:
Glucose absorbance as a function of wavenumber is shown in Figure 2 for seven different concentrations of glucose in distilled water, ranging from 1mg/mL to 100mg/mL. The chart is centered on a distinct glucose absorption peak at 1085 cm-1. Comparing the absorbance at this peak provides a sensitive and reliable metric for detecting changes in glucose concentration. As expected, absorbance increases proportionally with glucose concentration. These measurements were taken with an optical path length of 25 µm through the liquid cell. With this configuration, the signal-to-noise ratio becomes insufficient to reliably detect glucose below 5 mg/mL.
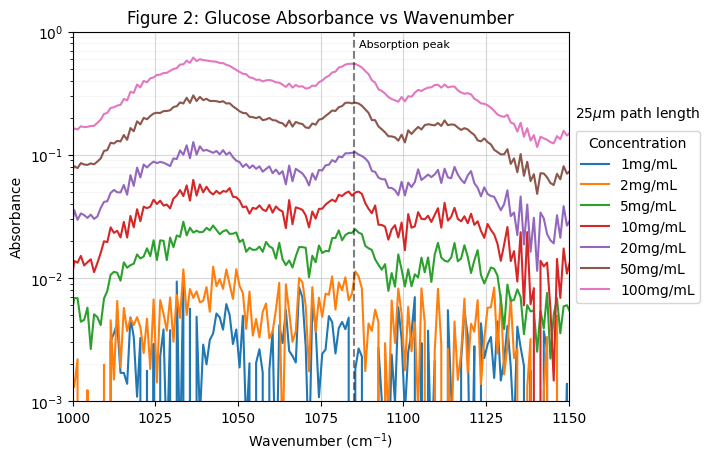
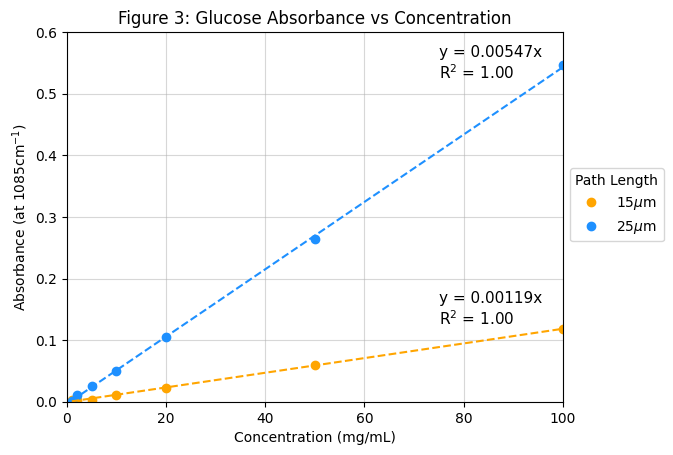
Figure 3 shows the absorbance of glucose at its absorption peak of 1085 cm-1 plotted as a function of concentration for two different optical path lengths: 25 µm (blue) and 15 µm (orange). Both datasets exhibit strong linearity consistent with the Beer-Lambert law, having R2 values of 1.00. The linear relationships confirm the ability to quantify glucose concentration levels through absorbance measurements. The 25 µm path length exhibits a steeper slope than the 15 µm path length, indicating higher sensitivity. A longer path length increases the probability of molecular interaction between the glucose molecules and the infrared beam, resulting in greater light absorption. This enhancement improves detection sensitivity, particularly at lower concentration levels. In a static environment, this path length can be optimized to the desired concentration ranges to enable detection of lower levels while still maintaining signal strength at higher levels.
Similar investigations were conducted for fructose, lactose, and glutamine at varying concentrations ranging from 1 mg/mL to 100 mg/mL (note: glutamine's solubility in water is about 25 mg/mL). Each of these chemicals were analyzed using a 25 µm optical path length due to its increased sensitivity. It was found that these chemicals also exhibit strong linearity between absorbance and concentration. The wavenumbers of the selected absorption peaks are displayed in Table 1 alongside their corresponding R2 linear fit values. The strong linearity demonstrates proof of concept for not only these selected chemicals, but also for other compounds with distinct absorption features within Block's current QCL tuning range of 800 - 1800 cm-1.
Table 1: R2 values for absorbance vs concentration linear fits
| Chemical | Absorption peak investigated | R2 of linear fit (25 µm path length) |
|---|---|---|
| Glucose | 1085 cm-1 | 1.00 |
| Fructose | 1070 cm-1 | 0.993 |
| Lactose | 1080 cm-1 | 0.999 |
| Glutamine | 1410 cm-1 | 0.993 |
Conclusion:
Block Engineering's QCLs have demonstrated effectiveness in quantifying chemical quantities in liquids due to their high sensitivity and spectral resolution capabilities. They offer greater sensitivity than Raman spectroscopy in a non-contact method that ATR-FTIR cannot achieve. They are also capable of real-time, in situ monitoring that other methods such as chromatography and mass spectrometry are incapable of. Operating over the infrared "fingerprint region," Block's QCLs can target many bioreactor-relevant chemicals with unique absorption features.
The absorbance spectra of the target analytes collected via QCLs showed strong linear correlations with concentration. This supports the concept of accurately determining chemical concentration levels by measuring absorbance in a static and repeatable environment.
The glucose concentration limit of detection with a 25 µm path length in this configuration was approximately 5 mg/mL, and a 15 µm path length resulted in a greater detection threshold. This demonstrates that path length can be optimized for a desired concentration range by detecting lower levels while maintaining sufficient signal at higher levels. Additionally, the use of flowing water — rather than static water, as used in these investigations — may further improve signal strength by increasing molecular interactions with the infrared beam and reducing noise.
A dedicated liquid transmission spectrometer designed for in situ monitoring could allow for continuous water flow during measurements. This would enable high sensitivity live monitoring of bioreactors in situ in a non-contact, non-destructive way.
Please contact us to discuss how Block's quantum cascade lasers can assist in your applications.

